

The dissolved substances in a solution are called solutes. The chemical reactions of life take place in aqueous solutions.
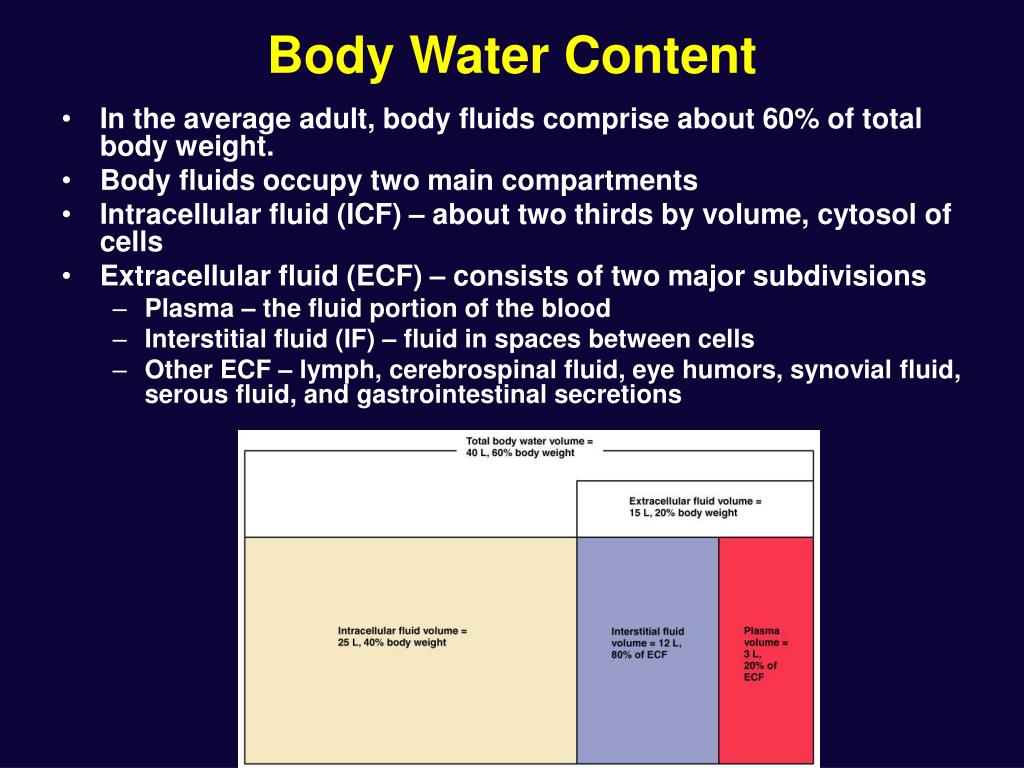
Body fluid compartments percentage free#
Thus there isn’t very much free magnesium in the actual cell water its all complexed
Body fluid compartments percentage plus#
Plus magnesium, like calcium, binds to the phosphorylated groups of the cell wall lipids, and acts as a membrane stabilizer. Those enzymes require magnesium to function. Intracellular magnesium is bound to ATP, cell wall lipids and many various enzymes.
Changes in the ECF magnesium concentration result in a slow change of intracellular magnesium concentration. Doesn’t look like there is any sort of transporter. Magnesium enters cells freely, without the need for active transport.īut we still don’t know how. There isn’t enough of it to matter seriously in any of the Gibbs-Donnan effects, or to contribute significantly to the various osmolar forces. Magnesium equilibrates freely across the extracellular fluid

500mmol is exchanged between bone and ECF over a 24 hr period Of this, over 99% is locked up in bone.A 70kg male has about 25 moles of calcium, or about 1000g.You have about 360mmo/kg of calcium in your body that makes about 25 moles of calcium.Cells differ somewhat in their potassium content, and this gives rise to a confusing plethora of values one can find quoted in the physiology textbooks (from 120 to 150mmol/L) Total body calcium content According to Vander's Renal physiology the skeletal muscle contains most of one's potassium stores, because it contributes the largest intracellular volume to the overall count. One's intracellular stores of potassium are not distributed as homogeneously as the shiny purple-filled cylinder would have you believe. Potassium concentration inside the cell is kept artificially high by the action of Na+/K+ ATPase, which exchanges 3 sodium atoms for every 2 potassium. Potassium equilibrates freely and rapidly across the extracellular fluid Extracellular fluid contains 2% of the total body potassium, and bone contains 8%.Of this, 90% is in the intracellular fluid.A 70kg male has about 2800mmol, or about 109g of potassium. Sodium concentration inside the cell is kept artificially low by the action of Na+/K+ ATPase, which exchanges 3 sodium atoms for every 2 potassium.
An equilibrium is reached where the sodium concentration in the plasma remains slightly higher, and the chloride concentration in the plasma is slightly lower (chloride ends up being higher in the interstitial fluid) Measuring the sodium content of plasma water alone would reveal a substantially higher concentration.Īnionic plasma proteins attract sodium into the plasma. BUT: there is actually more sodium in the plasma, because it is attracted there by the Gibbs-Donnan effect of all those anionic plasma proteins. When plasma sodium is measured, typically the concentration is not much different than in the interstitial fluid. Intracellular fluid contains 5% of the total body sodium.Extracellular fluid contains 50% of the total body sodium.Of this, 70% is “exchangeable” and the rest is locked up in bone crystal.A 70kg male has about 4200mmol, or about 92g of sodium.


 0 kommentar(er)
0 kommentar(er)
